In The News

Published July 22, 2024 | By O2 SEO Developer | Home Page
This Q&A style-article takes a deep dive into insights on cancer care for pets
Exploring the future of veterinary oncology, Neal Mauldin, DVM, ACVIM, ACVR, Thrive Pet Healthcare’s national director of radiation oncology and chief medical officer for Petcure Oncology, and Julie Bulman-Fleming, DVM,... Continue reading
Published April 13, 2024 | By O2SEO | Home Page
Advancements in veterinary radiation therapy are transforming cancer care for animals, bringing new hope and possibilities for their wellbeing. Offering increased precision with significantly reduced side effects, patients can maintain an excellent quality of life while achieving equivalent or better... Continue reading
Published April 5, 2024 | By O2SEO | Home Page
Former Animal Emergency Hospital of Mansfield expands services and rebrands
April 1, 2024 (MANSFIELD, Texas) – Today, Thrive Pet Healthcare Specialists Mansfield, formerly Animal Emergency Hospital of Mansfield, has expanded its services to include medical oncology and radiation oncology, providing... Continue reading

Published October 25, 2023 | By Lauren | In The News
October 24, 2023 (CHICAGO) – Thrive Pet Healthcare, a first-of-its-kind veterinary healthcare network with more than 380 locations in 37 states across the U.S., is proud to announce the opening of Thrive Pet Healthcare Specialists in Hoffman Estates. This multi-discipline veterinary facility offers the... Continue reading
Published September 25, 2023 | By Lauren | Blog
If you're a cat owner, you understand the deep bond you share with your feline friend. However, when our beloved cats suffer from chronic conditions like cystitis, it can be heart-wrenching and frustrating. We witness their discomfort and pain, feeling helpless at times.
Fortunately, a promising new... Continue reading

Published October 5, 2022 | By PetCure Oncology | In The News
Cats are known as finicky eaters. Even when they are in optimal health, many tend to be picky about their diets, turning their back on foods that are just not interesting enough. When a cat is diagnosed with cancer, getting him or her to eat can be even more challenging. Yet at the same time, it becomes more... Continue reading

Published September 19, 2022 | By PetCure Oncology | In The News
Good nutrition is undeniably one of the keys to a dog’s health. So, it should come as no surprise that a proper diet is even more crucial when a dog is fighting cancer. That raises an obvious question for pet parents: What is a good diet for a dog with cancer?
Unfortunately, there is no single or simple... Continue reading

Published September 7, 2022 | By PetCure Oncology | In The News
Getting a cancer diagnosis for your beloved cat or dog can launch a journey filled with emotional peaks and valleys. After the initial difficult news, your hopes may get a lift if your veterinarian reports that there’s a treatment course that can extend your pet’s life, improve his or her quality of... Continue reading

Published July 27, 2022 | By PetCure Oncology | In The News
Whether you cherish a Chihuahua, adore an Akita or are partial to a pooch of a different breed, there are a few basic things you want to give your dog. A happy home. The perfect name. Most important, the healthiest life possible.
One threat to canine health is cancer. More than 6 million dogs are... Continue reading

Published July 27, 2022 | By PetCure Oncology | In The News
Did you know that having a cat in your home can be good for your health? Research has shown that owning a cat can lower your blood pressure and reduce your stress level. So, it seems only fair that — in return — you should help your cat live a long and healthy life. One way you can do that is by taking... Continue reading
Published July 26, 2022 | By O2SEO | Home Page
We are honored to have PetCure Oncology featured in the Washington Post. The writer, Kim Kavin gives an excellent and well-researched summary of the rising cost of veterinary medicine while sharing her testament to its effectiveness. She focuses on her personal story of how Stereotactic Radiation... Continue reading
Published June 25, 2021 | By Benjamin Chiswick | Featured Testimonials
A veterinary radiation therapist in Sugar Land got quite the surprise when the owner of one of her canine patients turned out to be a woman whose life she helped save back when she worked with humans.
This story first aired on 6/25/21 and was published online the same day at Fox 26... Continue reading
Published June 25, 2021 | By Benjamin Chiswick | Home Page
This article first appeared on 6/24/21 on Newsweek.com, written by Kate Fowler
Mollybear the dog has gone viral on TikTok, with more than 14.3 million views for a heartwarming video showing her undergoing radiation treatment for a nose tumor.
Pet Hero Molly Graney
The... Continue reading
Published December 10, 2020 | By Benjamin Chiswick | In The News
Cancer in the bones of animals has been around since the age of the dinosaurs. Today, large to giant breed dogs and older dogs tend to be diagnosed with bone cancer at a higher rate than smaller or younger dogs. Osteosarcoma accounts for 90-95% of canine bone tumors. Other bone tumors include chondrosarcoma,... Continue reading
Published November 30, 2020 | By Benjamin Chiswick | In The News
Brain and tumor are two words you never want to hear your veterinarian say in the same sentence.
But thanks to recent advances in veterinary cancer care, there are new ways we can help dogs with brain cancer. We truly live in a golden age of medicine where clinical capabilities are... Continue reading
Published May 4, 2020 | By Benjamin Chiswick | In The News
Sugar Land, TX – May 5, 2020 – COVID-19 has presented countless barriers to people and businesses around the world over the past few months, but it will not stop pets in the Houston area from gaining access to life-changing cancer care.
PetCure Oncology and Sugar Land Veterinary Specialists... Continue reading
Published April 20, 2020 | By Benjamin Chiswick | In The News
Any cancer diagnosis feels devastating. But there are ways to fight for your pet, and we are here to help you understand your options. The first step is to make sure you have the right information to make an informed decision. It’s important to have a veterinarian that you trust - whether they are your... Continue reading
Published April 10, 2020 | By Benjamin Chiswick | In The News
Nasal cancer is one of the most common forms of canine cancer - which also makes it one of the most commonly treated.
If your dog is diagnosed with a nasal tumor, or suspected to have one, your primary care veterinarian will likely refer you to a veterinary oncologist.
Can nasal cancer be cured?
The... Continue reading
Published March 26, 2020 | By Benjamin Chiswick | In The News
A national leader in advanced radiation oncology for pets, PetCure Oncology and its Clinical Team of Board-Certified Radiation Oncologists are providing enhanced remote visits at no cost during the COVID-19 pandemic
DEERFIELD, Ill., March 26, 2020 (Newswire.com) - PetCure Oncology, a leading national... Continue reading
Published March 24, 2020 | By Benjamin Chiswick | In The News
COVID-19 is Affecting the World including Our Pets
As the global pandemic has much of the country on an unprecedented lockdown, both people and pets are being affected in countless ways. Everyone wants to do the right thing and stay home to avoid spreading COVID-19. Non-essential businesses have been... Continue reading
Published October 22, 2019 | By Benjamin Chiswick | Home Page
... Continue reading
Published September 25, 2019 | By Benjamin Chiswick | In The News
Deerfield, IL – September 26, 2019 – National pet cancer care provider PetCure Oncology treated its 3,000th pet with advanced radiation therapy on Wednesday, reaching a historic milestone in the fast-growing field of veterinary radiation oncology.
The treatment was delivered to an... Continue reading
Published August 27, 2019 | By Benjamin Chiswick | In The News
Washington, DC – August 21, 2019 – Dr. Neal Mauldin joins The Pet Show with Dr. Katy - and his former student, Dr. Katy Nelson - to talk about cancer care for pets, including the most commonly diagnosed forms of cancer and the latest advances in radiation therapy.
Click here to watch!
RELATED: Mike... Continue reading
Published August 26, 2019 | By Benjamin Chiswick | In The News
Harrisburg, PA – August 25, 2019 – Dr. Neal Mauldin speaks about new cancer care technology for pets while presenting to the PVMA at the Keystone Veterinary Conference in Pennsylvania.
Click here for the news coverage on Harrisburg's Local21.
RELATED: Thyroid Cancer in Dogs: An Alternative... Continue reading
Published July 28, 2019 | By Benjamin Chiswick | In The News
Pittsburgh, PA – July 28, 2019 – Pittsburgh's Action News-4 visits PetCure Oncology at PVSEC to interview Melanie Addison, a human cancer survivor who is now seeking pet cancer treatment for her cat,... Continue reading
Published July 17, 2019 | By Benjamin Chiswick | In The News
Deerfield, IL - July 17, 2019 - PetCure Oncology, LLC, a company providing veterinary cancer treatment utilizing non-surgical stereotactic radiosurgery (SRS) and stereotactic radiation therapy (SRT), announced that it has engaged Boustead Securities, LLC, for a Regulation D 506(c) offering and other capital... Continue reading
Published December 26, 2018 | By Benjamin Chiswick | In The News
Cannelle, a 10-year-old Abyssinian cat, was named after the brownish-black color of her coat but her personality is the most distinctive thing about her. Both of her parents work from home, so she’s used to having people around and is very social and vocal. She goes to-and-from her cat towers and enjoys... Continue reading

Published November 30, 2018 | By Benjamin Chiswick | In The News
Osteosarcoma, a type of bone cancer, is the most common type of primary bone cancer in dogs, accounting for over 95% of all bone tumors. Other types of bone cancer include chondrosarcoma, fibrosarcoma, and hemangiosarcoma. “Primary” refers to cancer that starts in the bone versus spreading... Continue reading
Published November 7, 2018 | By Benjamin Chiswick | In The News
At PetCure Oncology, we deal with pet cancer every day. We work with the most experienced veterinary clinical teams, the best pet advocates to help coordinate care and have helped treat thousands of pets with cancer. November is Pet Cancer Awareness Month and we are sharing critical information all pet... Continue reading
Published November 5, 2018 | By Benjamin Chiswick | In The News
Life for Molly, a 13-year-old cat, is full of love and fun with her parents Glenna and John and pet siblings Sonny (3) and Russ (14). Her days mainly consist of eating and playing with her toys, but her little brother Sonny has brought new energy into the home and keeps everyone, including Molly, on their... Continue reading
Published October 26, 2018 | By Benjamin Chiswick | Home Page
Jacksonville, FL – October 26, 2018 – The PetCure Oncology team at Southeast Veterinary Oncology and Internal Medicine treated a white tiger from the Catty Shack Ranch in Jacksonville, FL. Meet the amazing team, led by radiation oncologist Dr. Tracy LaDue, and learn more about Hercules'... Continue reading
Published September 26, 2018 | By Benjamin Chiswick | In The News
Life for four-year-old Othello had been rough but was made sweeter through the love of his mom Shaina. Shaina got him as a puppy when he was just 12-weeks-old in 2014 and she’s loved and cared for him ever since.
In his free time, he loves to play with bones, play with his pet siblings Quazar, Mozart,... Continue reading
Published September 11, 2018 | By Benjamin Chiswick | In The News
The word "cancer" is one of the hardest words to hear as a pet owner. Your pet is a member of your family, and their health and comfort are important to you. To give them the best care and make the most appropriate treatment decision, you need to have all the facts. It’s important for you to be able to... Continue reading
Published August 30, 2018 | By Benjamin Chiswick | In The News
Pittsburgh (August, 2018)— A new, innovative and effective cancer treatment is helping save the life of the Pittsburgh Zoo & PPG Aquarium’s 23-year-old California sea lion, Zoey.
Through the Zoo’s partnership with the Pittsburgh Veterinary Specialty and Emergency Center (PVSEC) and PetCure... Continue reading
Published August 28, 2018 | By Benjamin Chiswick | In The News
Chicago, IL – August 28, 2018 – PetCure Oncology and South Carolina Veterinary Specialists & Emergency Care (SCVSEC) will discontinue their veterinary radiation therapy program, effective September 2018. The partnership, which established the first clinic to feature stereotactic radiation (SRS/SRT)... Continue reading
Published August 13, 2018 | By Benjamin Chiswick | In The News
As a cat owner, you know when your cat is happy or hungry, but would you be able to tell if your cat was in pain? It can be difficult to tell if your cat is in pain, especially if your cat is very independent. Knowing the key signs of pain can help your pet avoid further injury or illness and help give you... Continue reading
Published August 13, 2018 | By Benjamin Chiswick | In The News
You know if your dog is happy or hungry, but how do you know if it is in pain? As pet parents, we want to make sure our dogs are living their best lives and pain can be difficult to determine in your pet. Knowing the key signs of pain can help your pet avoid further injury or illness and help give you peace... Continue reading
Published August 3, 2018 | By Benjamin Chiswick | In The News
Jacksonville, FL - August 3, 2018 - First Coast News (NBC-12) in Jacksonville goes behind the scenes with Dr. Tracy LaDue, a cancer survivor, and PetCure Oncology at... Continue reading
Published July 26, 2018 | By Benjamin Chiswick | In The News
Your cat isn’t just a furry, friendly roommate, your cat is a beloved member of your family. When a cat is diagnosed with cancer, it can be devastating. Did you know that more than 6 million cats are diagnosed with cancer every year? Cancers, like lymphoma, are even more prevalent in cats between the ages... Continue reading
Published July 26, 2018 | By Benjamin Chiswick | In The News
The Top Dog Beaches to Take Your Pet This Summer
Summer is here! And if you’re traveling to a PetCure Oncology location, these are a few dog beaches your pet will love. There’s no better way for your pet to celebrate their new role as a Pet Hero than running along the waves and getting some fresh air.... Continue reading
Published July 23, 2018 | By Benjamin Chiswick | In The News
Dog Breeds & Cancer
While it is important to note that any dog can get any type of cancer, there are certain breeds that are diagnosed with cancer at a higher rate than others.
Golden retrievers - The two most common types of cancer in this breed are hemangiosarcoma and... Continue reading
Published June 15, 2018 | By Benjamin Chiswick | In The News
Life as an 8-year-old German Shepherd on the sunny coast of Florida was exactly what you’d expect for Bella. Her days were full of fun in the sun with her dad, Dave Wright, and hanging out by the pool, swimming at the beach, and playing catch with her dog sister, Sasha.
“Bella really is a man’s best... Continue reading
Published June 11, 2018 | By Benjamin Chiswick | In The News
June 18th is Veterinarian Appreciation DayTM, and PetCure Oncology would like to celebrate all the hard work, dedication and compassion that veterinarians put forth for their pet patients and their families.
Help PetCure Oncology celebrate your veterinarian by nominating a veterinary professional that has... Continue reading
Published April 20, 2018 | By Benjamin Chiswick | In The News
Earth Day is April 22 and there’s no better way to celebrate than to enjoy some quality time outdoors--with your pet! Here are a few activities that can help you get in the Earth Day spirit and make some great memories with your dog or cat.
Tidy Up the Dog Park
If your neighborhood has a dog... Continue reading
Published February 14, 2018 | By Benjamin Chiswick | In The News
Clifton, NJ - February 14, 2018 - PetCure Oncology in Clifton, New Jersey gets a visit from Live it Up with Donna Drake and Pet Correspondent Robbyne... Continue reading

Published January 25, 2018 | By PetCure Oncology | In The News
Paddy, an orange and white Tabby, is a prominent member of Christine Ford’s family. He was first a companion for Christine’s mother and sister. After they both passed away, Paddy became a comforting friend for Christine. She vowed to take great care of her feline friend.
Signs & Symptoms
In... Continue reading

Published January 15, 2018 | By PetCure Oncology | In The News
Your pet is a member of your family, and their health and comfort are important to you. Learning that your pet has cancer can be a devastating and tumultuous experience - we know, we’ve been through it! It’s hard to know where to turn to find the right information on your pet’s cancer type, treatment... Continue reading

Published December 28, 2017 | By PetCure Oncology | In The News
This year we are grateful for many things, but most important to us is the opportunity to improve the lives of so many pets and their families with revolutionary cancer treatment. Since our launch in 2015, we have treated over 1,300 patients! That’s 1,300 families who gained more time, more love, and more... Continue reading
Published December 15, 2017 | By Benjamin Chiswick | In The News
Phoenix, AZ - December, 2017 - PetCure Oncology's innovative treatment of a jaguar from the Phoenix Zoo draws the attention of local TV news... Continue reading
Published December 11, 2017 | By PetCure Oncology | In The News
Jaguar at the Phoenix Zoo Receives Cutting-Edge Liquid Fiducial Cancer Treatment
PHOENIX, Ariz., Dec. 11, 2017 – PetCure Oncology announced the treatment of Caipora (ky-POUR-uh), a 12-year-old female jaguar at the Phoenix Zoo. The combination cancer treatment utilized liquid fiducial tissue marker,... Continue reading

Published December 11, 2017 | By PetCure Oncology | In The News
The holiday season is a fun and festive time of year, but some of our favorite traditions can pose a danger to pets. For a pet fighting cancer, the risks for injury, illness, or complications can be greater. Follow these tips for a safe and happy holiday celebration for all.
Anchor Christmas Trees to... Continue reading
Published December 7, 2017 | By PetCure Oncology | In The News
Chicago, IL and Copenhagen, Denmark – December 7, 2017 – PetCure Oncology Inc. (PetCure) and Nanovi A/S (Nanovi) announced an exclusive agreement to use PetXmark™ in the development and commercialization of fiducial guided Stereotactic Radiotherapy (SRT) for dogs and cats. PetCure is the leading... Continue reading

Published November 27, 2017 | By PetCure Oncology | In The News
With the holidays officially upon us, we’re coming up with new ways to spread holiday cheer and give back to animal-loving organizations throughout our community. Our commitment to saving the lives of pets goes far beyond the cancer care we provide. It’s the driving force behind our... Continue reading

Published November 16, 2017 | By PetCure Oncology | In The News
October was a busy month for the PetCure Oncology team!
In addition to revolutionizing cancer care for cats and dogs, PetCure Oncology is committed to working with veterinarians as they begin using stereotactic radiation (SRS/SRT) as a new cancer treatment option. PetCure Oncology participated in three... Continue reading
Published November 13, 2017 | By PetCure Oncology | In The News
Columbia, SC – November 13, 2017 – PetCure Oncology at South Carolina Veterinary Specialists & Emergency Care (SCVSEC) is officially open and accepting patients, marking the arrival of a new, potentially life-saving cancer treatment for pets in and around South Carolina.
This advanced form of... Continue reading
Published November 6, 2017 | By Benjamin Chiswick | In The News
Clifton, NJ - November 6, 2017 - PetCure Oncology at VRIC is highlighted in a national CBS news story about Pet... Continue reading

Published October 26, 2017 | By PetCure Oncology | In The News
Traveling with pets can be difficult enough, but when traveling for cancer treatment there are additional steps to take. You want to ensure your pet is as relaxed and comfortable as possible. Keep the following tips in mind while packing and traveling.
Bring the Essentials
Medications – If your pet... Continue reading
Published October 17, 2017 | By Benjamin Chiswick | In The News
Glendale, WI - October 17, 2017 - The Morning Blend segment on Milwaukee's WTMJ (NBC-4) visits Dr. Kelsey Pohlmann at PetCure Oncology's location in southeast... Continue reading

Published October 10, 2017 | By PetCure Oncology | In The News
When the weather starts getting cooler, there are a few steps you need to take in order to keep your pets safe. Just as your body adapts to the cold weather, your pet’s does too. Follow these quick tips to keep your pets safe this winter season.
If you are cold, your pet probably is too
Many people... Continue reading
Published September 22, 2017 | By PetCure Oncology | Home Page
When pet owners hear that their beloved animal has cancer, they are left with few options beyond euthanasia or drawn-out painful methods that may not guarantee a pet will live healthy or much longer.
However, SAGE, a veterinary specialty and emergency animal hospital in Campbell, believes there may be... Continue reading

Published September 7, 2017 | By PetCure Oncology | In The News
Most pet owners assume they would know if their pets were in pain. But most people don’t actually know the signs of discomfort in animals. And the other tricky thing is that dogs and cats tend to hide their pain. They are “wired” to do so because out in the wild, if they show weakness, they could be... Continue reading
Published September 7, 2017 | By PetCure Oncology | In The News
Campbell, CA – September 7, 2017 – It has been a historic week for PetCure Oncology, a national provider of cancer care for pets that treated it’s 1,000th patient on Tuesday.
Meet Peanut, the first Bay Area pet treated by PetCure Oncology... Continue reading
Published September 5, 2017 | By PetCure Oncology | In The News
Chicago, IL – September 5, 2017 – Since treating their first pet with cancer in 2015, PetCure Oncology has been working tirelessly to care for more pets - and a wider variety of pet cancers - than what was previously possible with traditional cancer care techniques.
[caption... Continue reading

Published August 28, 2017 | By PetCure Oncology | In The News
This past spring, we told you about a clinical trial launched by PetCure Oncology to study the use of stereotactic radiosurgery (SRS) in the treatment of lung tumors in dogs.
We already know that SRS has become a standard of care for treating lung tumors in human medicine. Most experts believe the... Continue reading

Published August 22, 2017 | By PetCure Oncology | In The News
If you’ve been to the San Francisco Bay Area, you know how time-consuming it can be to get to the South Bay from anywhere in the region. Still, every day, people make the trek to SAGE Campbell so their pets can receive conventional radiation therapy, which means 16 to 19 anesthetic events and treatments.... Continue reading
Published August 16, 2017 | By PetCure Oncology | Home Page
ORANGE PARK, FL – August 16, 2017 – PetCure Oncology at SEVO-Med is officially open and treating patients, marking the arrival of a new, potentially life-saving cancer treatment for pets in northeast Florida.
The PetCure Oncology at... Continue reading
Published August 8, 2017 | By Benjamin Chiswick | In The News
Jacksonville, FL - August 8, 2017 - PetCure's Dr. Tracy LaDue joins The Chat on Jacksonville's First Coast News (NBC-12) to explain veterinary radiation... Continue reading

Published August 4, 2017 | By PetCure Oncology | In The News
What an age of technology!
Twitters, tweets, emails, skype, texts, instant messenger, Facebook… and now I am on Slack! Who knew? Where do we draw the line between keeping up with the advancements and drowning in them?
Well, in an effort to keep up, I have taken my business and jumped into the deep end... Continue reading
Published July 27, 2017 | By PetCure Oncology | Home Page
PALO ALTO, Calif. — July 27, 2017 — According to the National Institutes of Health, an estimated six million dogs and six million cats are diagnosed with cancer each year in the US. Up to now the access to advanced treatments for these treasured family members has been limited. To increase veterinary... Continue reading
Published July 27, 2017 | By PetCure Oncology | Home Page
Campbell, CA – July 27, 2017 – Silicon Valley is no stranger to innovation. This August, pet owners in the Bay Area will benefit from the arrival of unprecedented cancer care technology for their furry loved ones thanks to a pair of strategic partnerships driven by PetCure Oncology, the... Continue reading

Published July 26, 2017 | By PetCure Oncology | Featured Testimonials
Across the country, stereotactic radiosurgery (SRS) is giving the families of pets with cancer the hope they are so desperately seeking.
Thanks to PetCure Oncology, more pet patients than ever now have access to innovative cancer care that is on par with the top oncology providers in human medicine.... Continue reading

Published July 18, 2017 | By PetCure Oncology | In The News
A type of bone cancer called osteosarcoma is the most common type of primary bone cancer, accounting for over 95% of all bone tumors. Other types of bone cancer are chondrosarcoma, fibrosarcoma, and hemangiosarcoma. “Primary” refers to cancer that starts in the bone versus spreading (metastasizing) into... Continue reading

Published June 20, 2017 | By PetCure Oncology | In The News
The dog days of summer will be upon us soon, and that means it’s time to brush up on our safety tips to keep your furry family member safe throughout the season. Every year, emergency rooms across the country receive many cases in which dogs and cats are suffering from heat stroke or burned paws, among... Continue reading
Published May 23, 2017 | By PetCure Oncology | In The News
Cincinnati, OH (FOX19) - Some people would do just about anything to keep their pets healthy and happy. Now, thanks to veterinary breakthroughs, even pets with difficult diagnoses can get the treatments they need to live longer. But, if you aren’t careful, that care could sink your budget. See this media... Continue reading

Published May 18, 2017 | By PetCure Oncology | In The News
We’re at it again!
In case you missed the news earlier this month, I’m excited to share the announcement that PetCure Oncology will be expanding into California and Florida later this summer. With new locations in San Jose, California and Jacksonville, Florida, stereotactic radiosurgery (SRS) will be... Continue reading

Published May 10, 2017 | By PetCure Oncology | In The News
When it comes to the safety of your loved ones, including your furry family members, it’s best to be prepared—really prepared. You can do many things before natural disaster strikes, and there’s no better time than now to put together a disaster plan for your pets.
Never Leave Your Pet at Home
If a... Continue reading
Published May 2, 2017 | By PetCure Oncology | In The News
Chicago, IL – May 2, 2017 – PetCure Oncology’s industry-leading national network of veterinary cancer care centers will continue to expand this summer with the addition of new sites in San Jose, California, and Jacksonville, Florida, the company announced on Tuesday.
The partnerships with SAGE Centers... Continue reading

Published April 26, 2017 | By PetCure Oncology | In The News
National Kids and Pets Day is celebrated on April 26 every year to recognize the special bond between children and pets. As veterinarians, technicians, and administrators working in the veterinary industry, we’ve all experienced the love of a pet. In fact, it’s probably safe to say that all of our... Continue reading
Published March 14, 2017 | By PetCure Oncology | Home Page
Chicago, IL – March 14, 2017 – In the world of pet cancer, lung tumors are a particularly nasty foe. But hope is on the horizon. PetCure Oncology, a national provider of cancer care for pets, is now recruiting candidates for a clinical trial intended to test a theory that has already been proven... Continue reading

Published March 13, 2017 | By PetCure Oncology | In The News
In the world of pet cancer, lung tumors are a particularly nasty foe. But hope is on the horizon.
PetCure Oncology is excited to announce our first clinical trial. It is intended to test a theory that has already been proven in human medicine:
Stereotactic radiosurgery (SRS) could be the answer for... Continue reading

Published March 1, 2017 | By PetCure Oncology | In The News
Every day, I see amazing things.
Beau was given three months to live before his pet parents learned about stereotactic radiosurgery (SRS). His human mom, Cindy, called Beau’s treatment and recovery “miraculous.” Snickers was on his way to be euthanized when his dad decided he couldn’t go through... Continue reading
Published February 28, 2017 | By PetCure Oncology | Home Page
Pittsburgh, PA (KDKA) – When you see 5-year-old Labrador retriever Beau today, it’s hard to imagine that just four months ago he was near... Continue reading
Published February 27, 2017 | By Benjamin Chiswick | In The News
Pittsburgh, PA - February 27, 2017 - Pittsburgh's KDKA (CBS-2) meets Beau and the Jagielski's after their experience with cancer treatment from PetCure Oncology at PVSEC in... Continue reading
Published February 17, 2017 | By PetCure Oncology | In The News
Blue Ash, OH —Twelve million dogs and cats are diagnosed with cancer every year. It's the leading non-accidental cause of death in pets. Now, a new tool is being used to save them, or at the very least prolong their life. It's available right here in... Continue reading

Published February 14, 2017 | By PetCure Oncology | In The News
Happy Valentine's Day! Today is the perfect day to shower our family and friends with expressions of love - and we can't forget about our pets.
Our pets provide us with unconditional love and deserve some extra affection on Valentine's Day. Every person has a unique relationship with their pets. To some... Continue reading
Published February 2, 2017 | By PetCure Oncology | In The News
Chicago, IL – January 31, 2017 – With an extremely busy year on tap, PetCure Oncology kicked off 2017 in style with the opening of its fifth site last week, PetCure Oncology at PVSEC in Pittsburgh, Pennsylvania.
Demitri received SRS at... Continue reading
Published January 24, 2017 | By PetCure Oncology | In The News
Pittsburgh, PA – January 24, 2017 – PetCure Oncology at PVSEC treated its first patient on Tuesday, officially marking the arrival of a new, life-changing cancer treatment for pets in western Pennsylvania.
Stereotactic radiosurgery (SRS) has emerged as a standard of care in human oncology over the last... Continue reading

Published January 19, 2017 | By PetCure Oncology | In The News
2017 is in full swing! The start of the year has been exciting and full of activity as we prepare for the launch of our new partner location in Pittsburgh, Pennsylvania. With all the buzz and excitement in the office and our centers, we wanted to take a moment and look at our personal goals for the... Continue reading

Published January 4, 2017 | By PetCure Oncology | In The News
On behalf of everybody here at PetCure Oncology, we would like to thank you for an amazing 2016 and wish you a happy and healthy 2017!
As we reflect on the first full year of the PetCure Oncology national network, we are immeasurably grateful for the kindness and support that pet owners across the country... Continue reading
Published January 1, 2017 | By North Hills Magazine (Pittsburgh) | In The News
Pittsburgh, PA - If you consider your pet a valued family member, it can be heartbreaking to learn that he or she has cancer. Yet researchers are developing innovative veterinary oncology treatment options to give pet owners cause for hope.
Original Story published in northhillsmonthly.com on January 1,... Continue reading
Published November 29, 2016 | By PetCure Oncology | In The News
Pittsburgh, PA – November 29, 2016 – Pet owners in and around Western Pennsylvania are just months away from the introduction of a new cancer treatment called stereotactic radiosurgery (SRS) to the market. The advancement will put them among only a handful of pet owners in the country with access to... Continue reading

Published November 23, 2016 | By PetCure Oncology | In The News
Thanksgiving is my favorite holiday. Perhaps that sounds trite, but it doesn’t make it any less true.
Come to think of it, I bet a lot of dogs out there feel the same way. I know that my dog, Bandit, always looks like he’s on top of the world on Thanksgiving. In addition to all of the attention he... Continue reading

Published November 9, 2016 | By PetCure Oncology | In The News
First, it’s important to understand that, like humans, pets can be treated for cancer, and sometimes even cured. Take a deep breath and get ready to arm yourself with knowledge—and support. After all, the cancer journey affects the entire family. Caregivers need support too!
Fortunately, there are a... Continue reading

Published November 3, 2016 | By PetCure Oncology | In The News
Cancer. What an ugly disease. Its insidious and devastating nature is unparalleled in today’s health care landscape.
But—believe it or not—I can think of one positive thing to emerge from this otherwise dreadful topic.
Unity.
History has shown that when people have their backs to the wall,... Continue reading

Published October 28, 2016 | By PetCure Oncology | In The News
At PetCure Oncology, our goal is to educate pet owners about pet cancer. In this article, we will address common rumors, myths, and the questions we hear from pet owners to get to the truth about pet cancer. Still, we can’t stress enough that you should always consult your veterinarian when it comes to... Continue reading

Published October 21, 2016 | By PetCure Oncology | Blog
Behind every great veterinarian is a great team of veterinary technicians.
That is why the veterinary industry designates one week every year to specifically recognize the invaluable contributions that vet techs make in the care of sick pets every single day.
Often working outside of the spotlight,... Continue reading
Published October 10, 2016 | By PetCure Oncology | In The News
Cincinnati, OH - October 10, 2016 – Pet owners in the Tri-State area have a lot to celebrate.
That will be on full display Thursday, October 13, when PetCure Oncology and Care Center celebrate the first anniversary of their partnership with an open house at Care Center’s 24/7... Continue reading

Published September 22, 2016 | By PetCure Oncology | Featured Testimonials
As my colleague Jack Moore blogged last week, PetCure Oncology’s approach to treating pet cancer is simple:
It is only and always about the pet family.
The root of that philosophy is that cancer, whether it is in a pet or a human, makes an impact far beyond the afflicted individual. Families navigate... Continue reading

Published September 20, 2016 | By PetCure Oncology | In The News
Is Pet Insurance Worth It?
This is a very common question from pet owners, and there is no “right” answer. But consider another question that may help you get to that answer:
If your pet experienced a medical emergency tomorrow, do you have the financial means to treat them?
As a veterinary cancer... Continue reading

Published September 9, 2016 | By PetCure Oncology | In The News
A cancer diagnosis is immobilizing. It may feel as if you went to sleep and awakened in a foreign land. The people are different. The language is different. The culture is different. The decisions you make and how you make them are different.
At PetCure Oncology, our focus on the pet patient and their... Continue reading

Published August 23, 2016 | By PetCure Oncology | In The News
In a previous pet cancer blog post, we discussed early warning signs of cancer to look for in your pet. So what happens when you notice one of those symptoms? The first step is to not hesitate or “wait it out.” If you notice anything irregular about your pet (physically, emotionally, or behaviorally),... Continue reading

Published July 13, 2016 | By PetCure Oncology | In The News
We were thrilled to read a great article on stereotactic radiation therapy, another common term that is used interchangeably with stereotactic radiosurgery (SRS), in this month’s issue of Trends Magazine. Trends is a premier monthly magazine published by The American Animal Hospital Association (AAHA) for... Continue reading

Published June 29, 2016 | By PetCure Oncology | In The News
Each year, about 12 million new cancer diagnoses are made in dogs and cats across the United States. Fortunately, we’ve seen great advancements in medicine over the years, which allow veterinarians to diagnose and treat cancer with greater success.
Early detection is crucial when it comes to pet... Continue reading

Published June 17, 2016 | By PetCure Oncology | In The News
The ACVIM Forum is one of the largest specialty conferences in the veterinary industry. Hosted by the American College of Veterinary Internal Medicine (ACVIM), this year’s conference was held last week at the Colorado Convention Center in Denver, Colorado. More than 3,000 veterinary professionals from all... Continue reading
Published May 31, 2016 | By PetCure Oncology | In The News
Chicago, Ill - May 31, 2016 - PetCure Oncology, the nation’s leader in advanced radiation therapy for pets, celebrated its one-year anniversary of treating patients this month. The first veterinary provider to make stereotactic radiosurgery (SRS) available to pets on a national scale, PetCure Oncology’s... Continue reading
Published May 26, 2016 | By PetCure Oncology | In The News
What a year it has been for the PetCure Oncology team.
Last Friday marked exactly one year since we treated our first patient, an inspiring golden retriever named Cienna. Barely a year later, I can’t help but reflect on how far we’ve come. It was not long ago that my family’s sweet dog, Juliette,... Continue reading

Published May 20, 2016 | By PetCure Oncology | In The News
I am going to be blunt. Brain tumors are terrifying.
I’m not really sure how else to say it. I could try to write something clever or witty or profound, but I would really just be beating around the bush. Any cancer diagnosis in our pets is distressing and brain cancer is no different. But there is... Continue reading
Published May 17, 2016 | By PetCure Oncology | In The News
Cincinnati, OH – May 17, 2016 – Brain Tumor Awareness Month comes along every May, providing the opportunity to reflect on how the perilous form of cancer impacts both humans and pets. This May, it holds extra meaning for Nancy and Craig Waikem, a mother and son from Massillon, Ohio, who are no strangers... Continue reading

Published May 11, 2016 | By PetCure Oncology | In The News
Clifton, NJ – May 11, 2016 –Steve Venier knows a thing or two about sick animals. As a regional manager for a large specialty and emergency animal hospital system, he has seen hundreds of ill and injured patients over the years. So when his own pet, a 10-year-old dog named Tina, was suddenly ailing, he... Continue reading

Published May 5, 2016 | By PetCure Oncology | In The News
Gilbert, AZ – May 5, 2016 – As the world celebrates National Brain Tumor Awareness Month this May, brain tumor survivor Calvin, an eight-year-old canine belonging to Gretchen and David May of Scottsdale, is happily playing with his favorite toy “crunchy bone” and chasing his French Bulldog sister... Continue reading

Published April 28, 2016 | By PetCure Oncology | In The News
PetCure Oncology celebrates World Veterinary Day each year. The holiday is held every year on the last Saturday in April.
At PetCure Oncology, we know that veterinarians are truly special people. They work tirelessly to provide medical care to animals. Their patients cannot verbally communicate where or... Continue reading

Published April 22, 2016 | By PetCure Oncology | In The News
I learn a lot of lessons from my 5-year-old daughter.
Gone are the days of keeping her happy with a poke of the belly or a sneaky game of peek-a-boo. She is growing up, after all, and quickly developing a discerning eye for dad’s bag of tricks.
Of course, that means she also knows the difference... Continue reading
Published April 15, 2016 | By PetCure Oncology | In The News
Mike Jones, R.T. (T), Director of Radiation Therapy at PetCure Oncology at Veterinary Radiosurgery and Imaging Center (VRIC) in Clifton, NJ, was a featured guest on a recent episode of Your Pet Matters by Dr. Michael Tokiwa.
Radiation Therapy with Mike Jones, R.T. (T)
The original podcast episode is no... Continue reading

Published April 13, 2016 | By PetCure Oncology | Featured Testimonials
We love hearing from our clients after their pets have received treatment at a PetCure Oncology cancer care center. Looking at the photos and video updates of their furry family members always puts a big smile on our faces. It reminds us of why we do what we do and renews our motivation to continue... Continue reading

Published March 18, 2016 | By PetCure Oncology | In The News
When I was a kid, there was a rotary phone in my room. Remember those? Cable television was a naïve dream for me. We had a functional record player in our house and a collection of records to go with it.
My, how times have changed.
My 5-year-old daughter has no idea what those things even are. Our... Continue reading
Published March 11, 2016 | By PetCure Oncology | In The News
First a radio station and now TV? Pet Hero Bindi is receiving a lot of attention and love for bravely fighting and beating cancer.
Bindi's owner, Mac Hussey shares Bindi's story to Sydney Benter of Local 12 WKRC in Cincinnati, Ohio in hopes that Bindi's story will help other families with pets diagnosed... Continue reading

Published March 4, 2016 | By PetCure Oncology | In The News
Does anybody else remember the show, “Where in the World is Carmen Sandiego?”
I used to play the computer games when home computers first became popular. Then I got into the game show when it came around. I always thought I could win if they ever let me on. I can still hear the catchy theme song in my... Continue reading
Published February 13, 2016 | By PetCure Oncology | In The News
Good news, everybody. 2016 is off to a great start!
Just before New Year’s, I contributed a blog to PetCureOncology.com that included one of my personal goals for 2016. I am committed to doing my part to raise awareness for an incredible new technology called stereotactic radiosurgery (SRS), which... Continue reading

Published December 29, 2015 | By PetCure Oncology | In The News
As we approach another New Year’s Eve, it is hard to believe how much has changed over the last year.
In March, I was intrigued when a friend of mine told me about a new professional opportunity she was considering. The company was intent on using advanced technology to fight – or even cure – cancer... Continue reading

Published December 21, 2015 | By PetCure Oncology | In The News
A 500-mile journey from Birmingham to Cincinnati saves Boxer from fatal brain tumor.
Cincinnati, OH — December 21, 2015 — What is the connection between a family in Birmingham, Alabama and a veterinary cancer care team in Cincinnati, Ohio, over 500 miles away? Addy, an 11-year-old Boxer diagnosed... Continue reading

Published December 18, 2015 | By PetCure Oncology | In The News
For many families, pets are an important and lovable member of the household.
Jill MacKenzie, program director for PetCure Oncology at Arizona Veterinary Oncology, knows this to be true when speaking about her Goldendoodle, Bella. That is why when Bella was diagnosed with soft tissue sarcoma (STS) in... Continue reading

Published November 25, 2015 | By PetCure Oncology | In The News
PetCure Oncology wishes all our furry friends, and their two-legged family members, a happy and healthy Thanksgiving. To keep the festivities healthy for the entire family, we’ve rounded up these Thanksgiving Pet Safety Tips.
Do you feel the need to share your family’s Thanksgiving feast with your... Continue reading
Published November 17, 2015 | By PetCure Oncology | In The News
Clifton, NJ - November 17, 2015 - Sandy Mainardi was devastated when Baci, her 13-year-old dog, began experiencing seizures, slipped into a coma, and was eventually diagnosed with a brain tumor. The prognosis was bleak.
Now, just 10 months later, the resident of Lincoln Park, New Jersey has seen her beloved... Continue reading
Published November 17, 2015 | By PetCure Oncology | In The News
When Mack Hussey and his family adopted their dog, Bindi, they had no idea that a small bump on her nose would quickly grow into a life-threatening cancer.
Fortunately, as the Hussey family learned, the conversation surrounding cancer care for pets is changing for the better – and fast.
Bindi’s... Continue reading
Published November 17, 2015 | By PetCure Oncology | In The News
Gilbert, AZ – November 17, 2015 – Jill MacKenzie spends her days helping veterinary professionals treat pets with cancer, but her personal and professional lives intersected unexpectedly when her nine-year-old Goldendoodle, Bella, was diagnosed with a soft tissue sarcoma last month.
Now, Bella is... Continue reading

Published October 27, 2015 | By PetCure Oncology | In The News
October 31st marks the eve of All Saint’s Day, celebrated by dressing in costume and going door-to-door to collect candy and treats. This can be a fun family event and with a little planning and preparation can also include our furry, four-legged family members.
Secure Pets When Trick-or-Treaters Come... Continue reading
Published October 20, 2015 | By PetCure Oncology | In The News
Chicago, IL - October 20, 2015 - PetCure Oncology, the leader in providing cancer care for pets, is pleased to announce the selection of Radialogica’s fullAccess™ software as the company’s platform for clinical collaboration and quality management. PetCure Oncology formally implemented fullAccess™... Continue reading
Published October 13, 2015 | By PetCure Oncology | In The News
Clifton, NJ – October 13, 2015 – PetCure Oncology at Veterinary Radiosurgery & Imaging Center (VRIC) recognizes the special contributions made by veterinary technicians on a daily basis. These exceptional individuals play a significant role in the health and well-being of pets, ensuring compassionate... Continue reading
Published October 13, 2015 | By PetCure Oncology | In The News
PetCure Oncology is paying tribute to the special efforts made by veterinary technicians every day. Similar to nurses in human healthcare, technicians play a vital role in the health and well-being of patients, providing attentive care and extrordinary compassion to our furry family members.
National... Continue reading

Published October 12, 2015 | By PetCure Oncology | In The News
This week the team at PetCure Oncology celebrates National Veterinary Technician Week, paying tribute to the special efforts made by veterinary technicians on a daily basis. We cover a day in the life of a vet tech, reasons to say thank you, a personal letter from a veterinarian and share career information... Continue reading
Published October 7, 2015 | By PetCure Oncology | In The News
Pioneers in veterinary and human oncology come together to help revolutionize cancer care for pets
Chicago, IL – October 7, 2015 – PetCure Oncology, the leader in cancer care for pets, is proud to announce that its newly-created Scientific Advisory Board (SAB) recently met for the first time and... Continue reading

Published September 30, 2015 | By PetCure Oncology | In The News
This week the team at PetCure Oncology celebrates autumn with tips for keeping your pets healthy as the weather turns crisp and cool. Read on and you’ll find suggestions on preventing ticks, averting Halloween candy hazards, avoiding snakebites and limiting holiday plant dangers.
- The team at Catster... Continue reading
Published September 23, 2015 | By PetCure Oncology | In The News
Revolutionary stereotactic radiosurgery treatment offers new hope for survival and quality of life
Chicago, IL – September 23, 2015 – Prostate cancer is among the most common cancers in American men, but it can be just as devastating for male dogs, accounting for as much as one percent of all... Continue reading

Published September 21, 2015 | By PetCure Oncology | In The News
This week the team at PetCure Oncology shares a list of resources to help pet parents navigate a cancer diagnosis in their four legged family members. The list covers new terminology you may hear when talking with your veterinarian, getting a second opinion, managing your emotions, conventional and holistic... Continue reading
Published September 16, 2015 | By PetCure Oncology | In The News
Infusion of additional resources will continue to help revolutionize cancer care for companion animals.
Clifton, NJ – September 16, 2015- Veterinary MRI and Radiotherapy Center of NJ, a pioneer in providing leading-edge cancer care and imaging services to the region’s companion animals, has been... Continue reading
Published September 15, 2015 | By PetCure Oncology | In The News
We are pleased to share with you the news that PetCure Oncology has acquired Veterinary MRI and RT Center of New Jersey in Clifton. The practice has been renamed to PetCure Oncology at Veterinary Radiosurgery & Imaging Center (VRIC).
PetCure Oncology was created by the founders... Continue reading
Published August 26, 2015 | By PetCure Oncology | In The News
Cincinnati, OH - August 26, 2015- Gus, a loveable, nine-year-old Golden Retriever mix, had the house to himself one afternoon when his owners were out running errands. While they were gone, Gus had a bloody nose. By the time they returned, there was blood all over the living room floor. He had been a... Continue reading
Published August 22, 2015 | By PetCure Oncology | In The News
Treating cancer in a pet is hard. It's hard on the animal and on the owners watching their pets suffer and paying the bill. It's also hard to find alternatives to radical surgery.
Scott Milligan is trying to make battling four-legged cancer easier. A former executive in the cancer-treatment industry,... Continue reading
Published August 19, 2015 | By PetCure Oncology | In The News
There is new hope for the 6 million dogs that are diagnosed with cancer each year. The latest veterinary cancer treatment is now available, a machine called Rapid Arc.
Reporter Jennifer Schack has the story...
RELATED: An... Continue reading

Published August 12, 2015 | By PetCure Oncology | In The News
Nothing is more heartbreaking than being the pet parent of a cat or dog diagnosed with cancer - just ask Cienna’s parents. At the end of last year Cienna began having seizures and was diagnosed with a brain tumor. As her family looked for answers they learned about a human cancer treatment that would soon... Continue reading
Published August 5, 2015 | By PetCure Oncology | In The News
Gilbert, AZ – August 5, 2015 – Cienna is a beautiful, six year old mixed breed dog. She was a very silly, playful dog until being diagnosed with a brain tumor, which made her lethargic and inactive. But 2 1/2 months ago, she received a revolutionary new treatment, and now she's back to chasing her... Continue reading
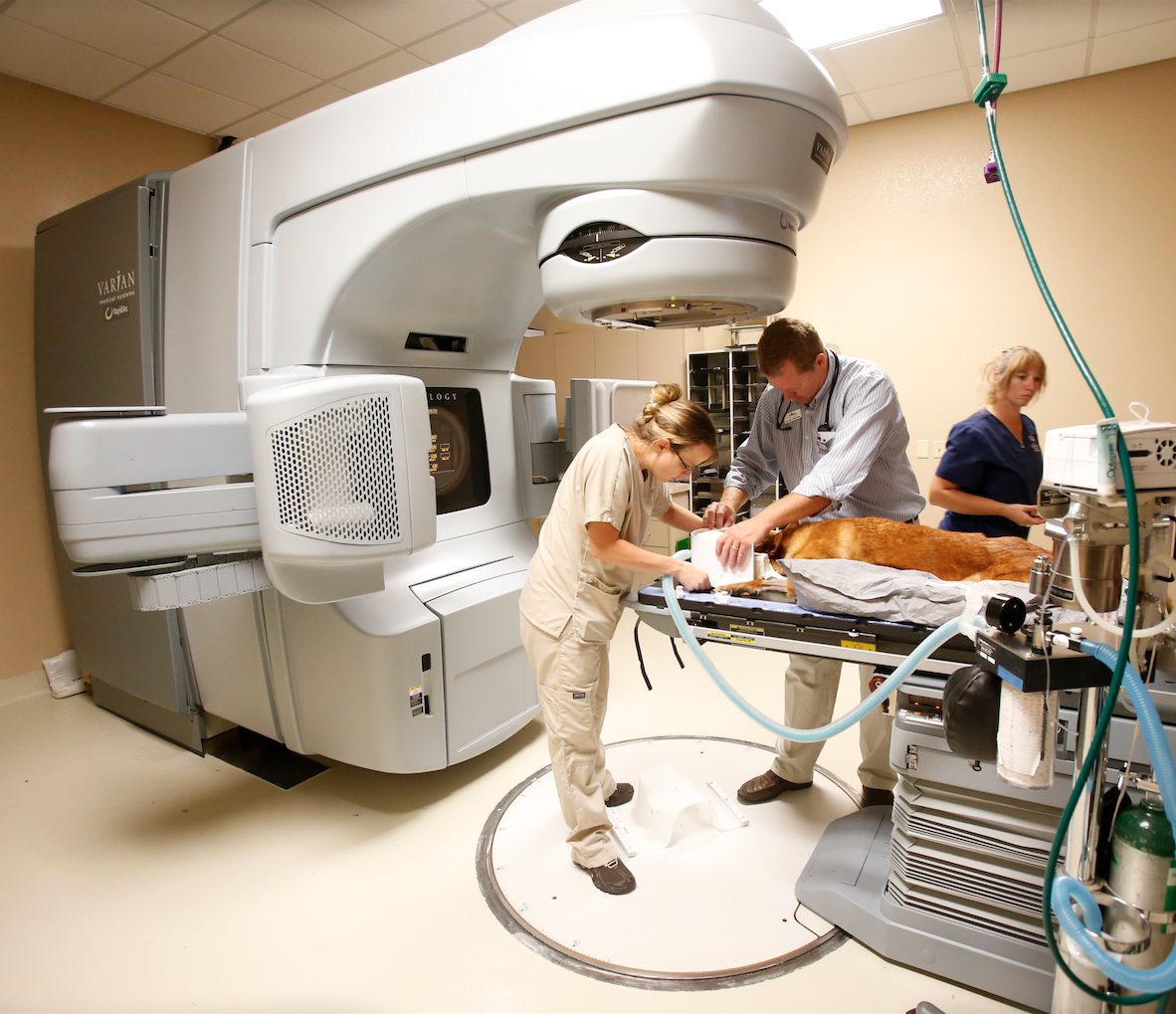
Published July 28, 2015 | By PetCure Oncology | In The News
Dr. Eric Boshoven of Arizona Veterinary Oncology shares his story on becoming the first location in the PetCure Oncology nationwide network of veterinary stereotactic radiosurgery centers. In his own words…
I became a veterinary radiation oncologist because I’m somewhat of a techno-geek that enjoys... Continue reading
Published July 16, 2015 | By PetCure Oncology | In The News
Chicago, Ill - July 15, 2015 - Pets are at risk of getting cancer just like humans. Of the estimated 70-80 million dogs and 74-96 million cats in the United States, cancer accounts for nearly 50 percent of all disease-related pet deaths and is the number one cause of death in older dogs and cats.
In... Continue reading
Published April 6, 2015 | By PetCure Oncology | In The News
Arizona Veterinary Oncology is thrilled to share with you the impending launch of one of the most exciting technological advances available in veterinary cancer treatment. Arizona Veterinary Oncology will be the first to make this new technology available in the Phoenix area, which will significantly improve... Continue reading
Published March 6, 2015 | By PetCure Oncology | In The News
The staff of Care Center (Cincinnati, Ohio) is thrilled to share the impending launch of one of the most exciting technological advances ever seen in veterinary cancer treatment. In fact, Care Center will be the first to make this new technology available in the Cincinnati area, which will significantly aid... Continue reading





























































